Each atom is composed of a central, positively charged nucleus—only a very small fraction of the atom's volume, but containing most of its mass—surrounded by a cloud of much lighter, negatively charged electrons. The number of electrons in an atom—ranging from 1 up to about 100—matches the number of charged particles, or protons, in the nucleus, and determines how the atom will link to other atoms to form molecules. Electrically neutral particles (neutrons) in the nucleus add to its mass but do not affect the number of electrons and so have almost no effect on the atom's links to other atoms (its chemical behavior). A block of pure carbon, for instance, is made up of two kinds, or isotopes, of carbon atoms that differ somewhat in mass but have almost identical chemical properties. Scientists continue to investigate atoms and have discovered even smaller constituents of which neutrons and protons are made. (Science For All Americans)
The Energy of 1 Nuclei Multiplied
Additional Resources:
US leaflets warning the people of Japan of impending atomic bomb.
Nuclear Non-Proliferation Treaty News (11 Jan, 2022)
Where is the Mass AND Energy?
The sum is not equal to the whole.
The inventor of the mass spectrometer, F. W. Aston discovered in 1920 the key experimental element in the puzzle. He made precise measurements of the masses of many different atoms, among them hydrogen and helium. Aston found that four hydrogen nuclei were heavier than a helium nucleus. This was not the principal goal of the experiments he performed, which were motivated in large part by looking for isotopes of neon.
source : dc.edu.au
The importance of Aston's measurements was immediately recognized by Sir Arthur Eddington, the brilliant English astrophysicist. Eddington argued in his 1920 presidential address to the British Association for the Advancement of Science that Aston's measurement of the mass difference between hydrogen and helium meant that the Sun could shine by converting hydrogen atoms to helium. This burning of hydrogen into helium would (according to Einstein's relation between mass and energy) release about 0.7% of the mass equivalent of the energy. In principle, this could allow the Sun to shine for about a 100 billion years. In a frighteningly prescient insight, Eddington went on to remark about the connection between stellar energy generation and the future of humanity:
If, indeed, the sub-atomic energy in the stars is being freely used to maintain their great furnaces, it seems to bring a little nearer to fulfillment our dream of controlling this latent power for the well-being of the human race---or for its suicide.
Splitting the nucleus
In 1932 Ernest Walton and John Cockcroft built a machine that achieved the first fully artificial nuclear reaction and nuclear transmutation. It was popularly known at the time as "splitting the atom". The photograph to the right shows Ernest Walton, Ernest Rutherford and John Cockcroft shortly after announcing the first ever splitting of the atom.
Protons were accelerated and directed at a sample of lithium which disintegrated into two helium atoms. The photograph below shows Walton sitting in a box at the bottom of the machine where he would observe scintillations (a flash or sparkle of light) on a screen.
The equation for the reaction is shown below.
The energy released in this reaction was of the order of 17 MeV. This is over 80 times the energy of the original bombarding proton. Measurement of the mass of the nuclei showed that about 2% of the mass had disappeared in the reaction. This was the first verification of Einstein's famous equation.
This equations suggest that energy and mass are equivalent and that mass can be formed out of energy and energy formed out of mass.
The gif below shows the reaction observed by Cockcroft and Walton.
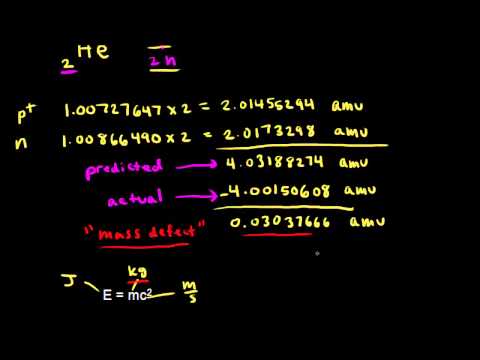
The nucleus requires binding energy to overcome the repulsion of positive charged protons. For example, the mass of a helium nucleus made of up two protons and two neutrons is slightly less than the sum of the masses of the four individual nucleons, as shown in the diagram below.
The difference in the mass is called the mass defect. In this case the mass defect is 0.0305 u. The unit here is the unified atomic mass unit (u).
Δm = ΔE c-2
1 u = 931.5 MeV c-2
This means that a mass defect of 1 u would have a binding energy of 931.5 MeV. This value is in the IB Physics Data booklet (pg. 1).
In the case of the helium nucleus above the binding energy E can be determined from the mass defect of 0.0305 u.
1 u = 1.661x10-27 kg (From the PDB)
ΔE = Δm c2
ΔE = 1.661x10-27 kg * (3.0x108ms-1)2
ΔE = 1.4949x10-10 J
1 J = 6.242x1012 MeV
or
1 MeV = 1.6022x10-13 J
1.4949x10-10 J *(1MeV/1.6022x10-13 J) ≈ 931.5 MeV
TOK: Why are these numbers approximations?
Binding Energy and Binding Energy per Nucleon
So the binding energy of helium-4 = 28.4 MeV.
The binding energy is the energy required to separate the nucleus into its component parts - or conversely the energy emitted when a nucleus is formed out of its component parts.
The binding energy per nucleon can be found by dividing the total binding energy by the number of nucleons. In the case of helium-4 the total binding energy should be divided by 4. Therefore the binding energy per nucleon of helium-4 is 7.1 MeV
The graph below shows the binding energy per nucleon plotted against mass number (or nucleon number).
The graph shows that helium-4 has an unusually high binding energy per nucleon compared to other nuceli of a similar size. The element with the highest binding energy per nucleon is iron.
TOK: 12C and 16O both lie outside the general curve of the graph, what does this say about Earth's biota and the search for life on other planets?
Nuclear fission
Watch the video clip where Jim Al Khalili explains the discovery of nuclear fission.
A typical fission of uranium is represented in the diagram where the uranium splits into barium and krypton.
Nuclear Fission Simulation
Make a copy HERE.
The gif below animates this reaction.
The sum of the mass of the products (the barium, krypton and 3 neutrons) is slightly less than the sum of the mass of the uranium and neutron to begin with. This mass defect accounts for the energy released in the reaction.
Choose Fission, One Nucleus and click on the red button to bombard the nucleus with a neutron.
Critical Mass of Fissible Material
We will use this to collect quantitative data critical mass/ assembly.
Nuclear Fusion
A typical fusion reaction is represented in the diagram where two isotopes of hydrogen (deuterium and tritium) fuse into helium and a neutron.
The gif below animates this reaction.
Current Research into Fusion Research:
Fusion Energy Overview
German Research
French Research
Resources:
For Reaction And Binding Energies and Activity Calculations in Nuclear Physics
Identify exactly what needs to be determined in the problem (identify the unknowns). This will allow you to decide whether the energy of a decay or nuclear reaction is involved, for example, or whether the problem is primarily concerned with activity (rate of decay).
Make a list of what is given or can be inferred from the problem as stated (identify the knowns).
For reaction and binding-energy problems, we use atomic rather than nuclear masses. Since the masses of neutral atoms are used, you must count the number of electrons involved. If these do not balance (such as in 𝛽+ decay), then an energy adjustment of 0.511 MeV per electron must be made. Also note that atomic masses may not be given in a problem; they can be found in tables.
Perform the desired calculation; keep careful track of plus and minus signs as well as powers of 10.
Check the answer to see if it is reasonable: Does it make sense? Compare your results with worked examples and other information in the text. (Heeding the advice in Step 4 will also help you to be certain of your result.) You must understand the problem conceptually to be able to determine whether the numerical result is reasonable.
Adapted from: OpenStax College Physics
Binding Energy Problem Sets
Binding Energy Group Problem
Using sum of the days you were born (i.e. 31 Oct, 11 May, and 04 August you would choose element 46 Palladium). For your element, please determine the following:
Atomic number
Atomic mass of the most stable isotope.
The Binding Energy per nucleon
State if the element undergoes fission or fusion.