Organic Chemistry Fundamentals
Before getting started with complex chemical reactions or structures, there are a few fundamental concepts that should be memorized prior to diving into a sea of organic chemistry problems.
The best practice method for this is to make physical flashcards or using online flashcards such as chegg flashcards. I personally liked chegg flashcards because they are free to use and can be taken ANYWHERE. The best part of it all...it's available on your phone, so whether your just walking across campus or taking a trip out of town you can use your flashcards anywhere!
-Allyssa Gomez, Chemistry Tutor
After reviewing naming, try some practice!
Ayssa Rivera, 2020
Structures
8/16/17 Shared by Lizbeth & Myrka
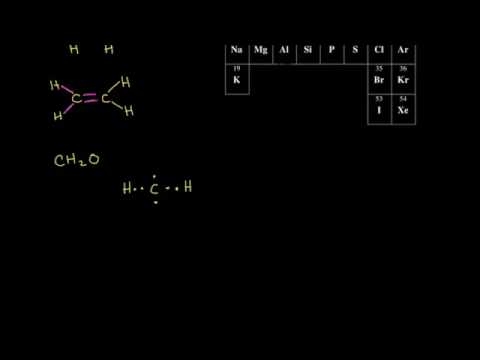
An array of structures can be formed from covalent bonds between carbon and other elements. This is an important fundamental concept to Organic Chemistry. Professors will see this as review from Chem 1A/1B (mostly 1A), so if you want more detailed information, please refer to those sections on this website .
Goals this week with the Chemistry tutors:
Translate dot structures to a stick structure
draw a compound given its molecular formula
be able to differentiate between functional groups
predict if a single or double bond will occur
discuss how to study for this topic
ask the tutor how to make flash cards for this subject
ask the tutor about making a list of notes in working on these problems
discuss how to take and study from practice tests
Activities to do at home:
Create flash cards
work on creating a list of the rules needed to finish these problems
summarize the lecture into 5 sentences
work on homework
use the provided links
Periodic Trends
Electronegativity
While you are no longer in a general chemistry course, there are a few concepts that will be carried over to chemistry 128A. Having a strong understanding of electronegativity will be essential prior to moving onto difficult concepts like resonance.
-Allyssa Gomez, Chemistry Tutor
Nomenclature
Before getting started, make sure you have reviewed and memorized the functiional groups under the "Organic Chemistry Fundamentals" tab.
What I enjoy about this tab is that it walks you through step by step in naming organic molecules. The way I studied was by making a large table with all the rules of nomenclature and practice lots of problems. Like they say . . . practice makes perfect!
-Allyssa Gomez, Chemistry Tutor
Practice Problems with Answer Key :
Nomenclature Rules and Guidelines :
NEWMAN PROJECTIONS
These are notes that will help you understand the steps in creating the Newman projections for different molecules. Being able to draw the conformations will help you see the positions of the groups and determine the stability of the molecule. If you need any assistance in answering the problems provided, stop by the Chemistry table at the Learning Center.
Myrka Macedo, Monthly Project #3, 4/25/18
This video is helpful for learning how to draw Newman projections. It also explains anti and gauche conformations and its relevance in determining relative energy between different staggered conformations
S and R Configurations
5. If the lowest priority IS in the back: The configuration you previously assigned is correct! Go ahead and leave the letter you wrote down. This is the configuration of that chiral carbon.
6. If the lowest priority is NOT in the back: Automatically switch your original noted letter to the opposite one. If you counted and found "R" for step 4, go ahead and change this to "S". The same thing goes for "S". If this was your original guess, switch it to "R". Whatever configuration you get after switching it will be the final one for this carbon.
added by Naomi Garcia, Fall 2024
How to Determine the Configuration of a Chiral Carbon
Step-by-step Guide:
Analyze the bond-line structure to identify each chiral carbon. Any carbon that has four different groups attached to it is chiral. Label these carbons with a star. Due to their chiral nature, they can be categorized into "S" and "R" configurations.
Focus on the chiral carbon, and circle the group that is all the way in the back. This group can be represented by a dashed line or may even be a hydrogen that is not drawn.
Ask yourself: Does this group have the lowest priority based on electronegativity or size? Usually, the lowest priority is assigned to hydrogens and simple methyl groups. Take a moment to number all four groups based on these rules.
Ignore this circled group and use the order/numbering of the other arms to determine if you are counting clockwise or counterclockwise. If you count the three arms clockwise, this is known as an "R" configuration. If it's counterclockwise, mark an "S" next to the carbon. Now, go back and analyze the circled back group.
Resonance Structures
Check out this page from Master Organic Chemistry for help with resonance structures.
It covers:
drawing valid resonance structures
determining the greatest contributors to the resonance hybrid
some practical applications of resonance structures
Test your knowledge using the quiz at the bottom of the page!
added by Magdalena, Fall 2024
Reactions
Predicting the Products of the Most Common Alkene Addition Rxns
This video is useful for those who need immediate review before an exam or those going into an a quiz or test without a clue what the reagents are for alkene addition reactions
Elimination Reactions
This video covers the types of compounds, the mechanisms, and the expected products of E1 and E2 reactions. Use the example problems at the end of the video to test your knowledge!
– Magdalena Hoskins, Spring 2024